
Electropolishing
Electropolishing: a superior finish
 Most austenitic steel alloys contain iron, 18% chrome and 8% to 10% nickel. The rich metals chrome and nickel are responsible for the chrome oxide skin which gives stainless steel a high corrosion resistance.
Most austenitic steel alloys contain iron, 18% chrome and 8% to 10% nickel. The rich metals chrome and nickel are responsible for the chrome oxide skin which gives stainless steel a high corrosion resistance.
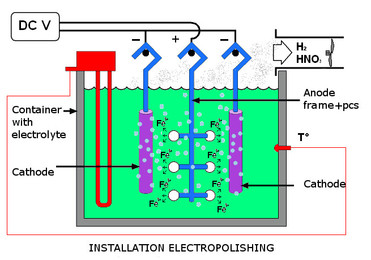
By electropolishing, which is an anodizing process where the treated objects are hung anodicly in baths filled with electrolyt and electric current. The chemicals dissolve the impurities and iron.
By this process, the ratio of chrome and nickel on the surface improves, making the stainless steel chemically very inert due to the development of a chromium oxide layer on the surface.
The advantages of electro polished surfaces are:
- Highly increased corrosion resistance
- Full recovery of the austenitic structure
- High resistance towards sticking
- Highly improved cleanability
- Very pure and hygienic and aseptic surfaces
- Smoother and more closed surface
- Elimination of welding discoloration, high brilliance
- Low electric resistance and good thermic conduction
- Halving the 3-dimentional surface
- Very passive and rich surface



